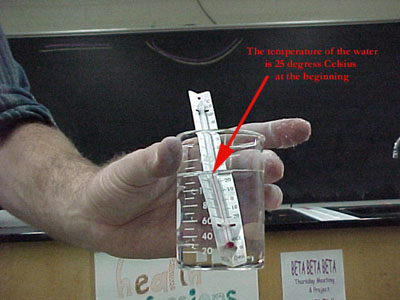
The water molecules have kinetic energy to begin with, but not enough to boil in the presence of air pressure. Some of the most energetic water molecules are leaving (evaporating), but this process is quite slow.

When we remove the air pressure, the most energetic water molecules become water vapor gas. This is the same reason it is easier to boil water in the mountains than it is at sea level: you need to add less energy to get the water boiling when there is less air pressure on it. Because water boils at a lower temperature in the mountains, we need to cook food longer for the same effect. Note: The bubbles in boiling water are not air, they are water vapor!

The boiling water did not get any extra energy, so its temperature didn't go up. In fact, since the most energetic molecules evaporated, the average kinetic energy of the ones left went down, and we read this as a decrease in temperature.
