
In the back of the book Science Instruction in the Middle and Secondary Schools (2002), the authors Eugene Chiapetta and Thomas Koballa suggest that "if you place a drop of water on a piece of wax paper and ask the question, Does the drop of water roll or slide across the paper? you will have a puzzle that needs to be resolved and explained." The book then goes on to describe how the exploration might proceed, explaining that some chalk dust or pepper in the drop will help students see that the drop rolls rather than slides. By way of explanation, the authors state that, "In this situation, we observe the cohesive effects of water molecules permitting a drop of liquid to form and maintain a spherical shape that will roll on a surface without breaking apart." What does this really mean? Is this explanation enough?
I decided to play around with this system and see what things I could come up with.
Let's start with the drop of water on the wax paper:

It is easy to see that the drop seems to have a "skin" holding it into
a sort of flattened sphere. It turns out that this surface tension
is the result of the tendency of water molecules to attract one another
(called cohesion). The lowest energy state for this drop occurs
when the maximum number of water molecules are surrounded on all sides
by other water molecules -- meaning that the drop should have the minimum
possible surface area, which is a sphere. The effect of gravity flattens
this ideal sphere into the shape we see.
Surface tension can be shown with many other demonstrations, including one in which you add coins to a full glass of water and note how the surface rises well above the rim of the glass:

Though teachers often talk about surface tension being like a "skin", this is not really an accurate model, as there is no other compound other than water in the glass. In addition, the surface does not react to being poked with a stick as a skin would. In fact, the water not only sticks to itself, it sticks to the wood as you can see by looking at the arrows in the picture. This is called adhesion because the attraction is to a different substance.
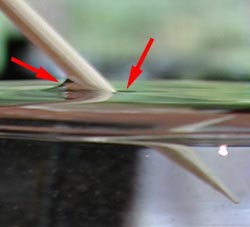
In all systems within which water interacts with another surface, both
adhesion and cohesion are factors. When cohesion is more of a factor,
the water forms spherical droplets; when adhesion is more of a factor,
we get sheets of water.

water drop on oiled plastic wrap (cohesion of water and only a little adhesion of water to plastic) |

water drop on untreated plastic wrap (cohesion of water molecules to one another as well as strong adhesion of water to plastic) |

Since the water drops stay on the wax paper, there must be some sort of attraction between these materials. It turns out the force of attraction between the water and the wax is actually quite strong, but it is less strong than the water-water (cohesive) force. For a good short discussion of a common misconception about hydrophobic materials, check out the Bad Chemistry page at http://www.princeton.edu/~lehmann/BadChemistry.html#hydrophobic.
On clean glass, the forces of adhesion between water and the surface are stronger than they are on oil or wax. The following image is of a mirror half of which I treated with butter (right) and half of which I left alone (left). I then put the mirror in the freezer to cool it and harden the butter, removed it, and dropped some water on it. Notice that the water on the left side (where cohesion and adhesion were both major factors) formed a sheet. On the right, where cohesion won out over adhesion, the water formed drops:

So now we have a mental model of what is going on with a drop of water
on a surface, and this should help us think about the original problem.
The water drop is close to spherical because of the cohesion of the water
molecules. (Gravity squashes it, however.) This ball of water, of course,
is also somewhat attracted to the wax paper (adhesion); thus, when it is
tilted, the surface of the water "sticks" to the wax, and the drop rolls.
For more discussion of intermolecular forces, a good webpage is Intermolecular
Bonding -- VAn Der Waals Forces, at
http://www.chemguide.co.uk/atoms/bonding/vdw.html
Now let's look at the glass again:

This principle is behind the trick of putting soap on your bathroom mirror to keep it from fogging. The fog on a mirror is caused by many tiny drops of water condensing on the mirror and reflecting light in a variety of directions. The soap disrupts the cohesion of the water molecules, causing the water to form sheets (through which the image can be seen relatively easily) rather than drops.
By the way, if we come back to our original drop of water on the wax paper, we can break the surface tension by adding soap, and we'll see that the drop changes into a flat puddle. Notice the relatively flat profile:

When we reduce the force of cohesion (by introducing soap), the drop
changes shape as now the forces of adhesion and cohesion are more nearly
the same.
One of the problems with an activity like the original water drop experiment is that some students feel like there is one right answer and stop wondering about the phenomenon once this answer is found. Moreover, many people will feel that finding out that the drop rolls is what this is all about, when this is really only the beginning. (In fact, even the book does a poor job explaining why it rolls and leaves one with more questions than answers.) A good teacher, by looking at a phenomenon more carefully herself before class, will have many questions and avenues of research for those students who get to a place where they need a challenge.
©2001 Jeff Goodman